Study evaluating the non-inferiority and better tolerability of the device PHOS-ISTOS® compared to the conventional photodynamic therapy (PDT) during the treatment in Actinic Keratosis (AK)
Ongoing / closed: Closed
Research type (rétro/prospective): prospective
Synopsis:
Actinic keratosis (AK) is a common chronic skin disease that can potentially progress to invasive squamous cell carcinoma (SCC). AK negatively affects the quality of life of patients with pain, itching or bleeding. On photodamaged skin, AKs are associated with subclinical lesions (cytological alterations) and sometimes with skin cancer, which has led to the concept of a cancer field.
Photodynamic therapy (PDT) is one of the treatments that has shown its effectiveness on clinical and subclinical dermatological lesions. In the literature, response rates between 70% and 90% are reported at 3 months after PDT treatment.
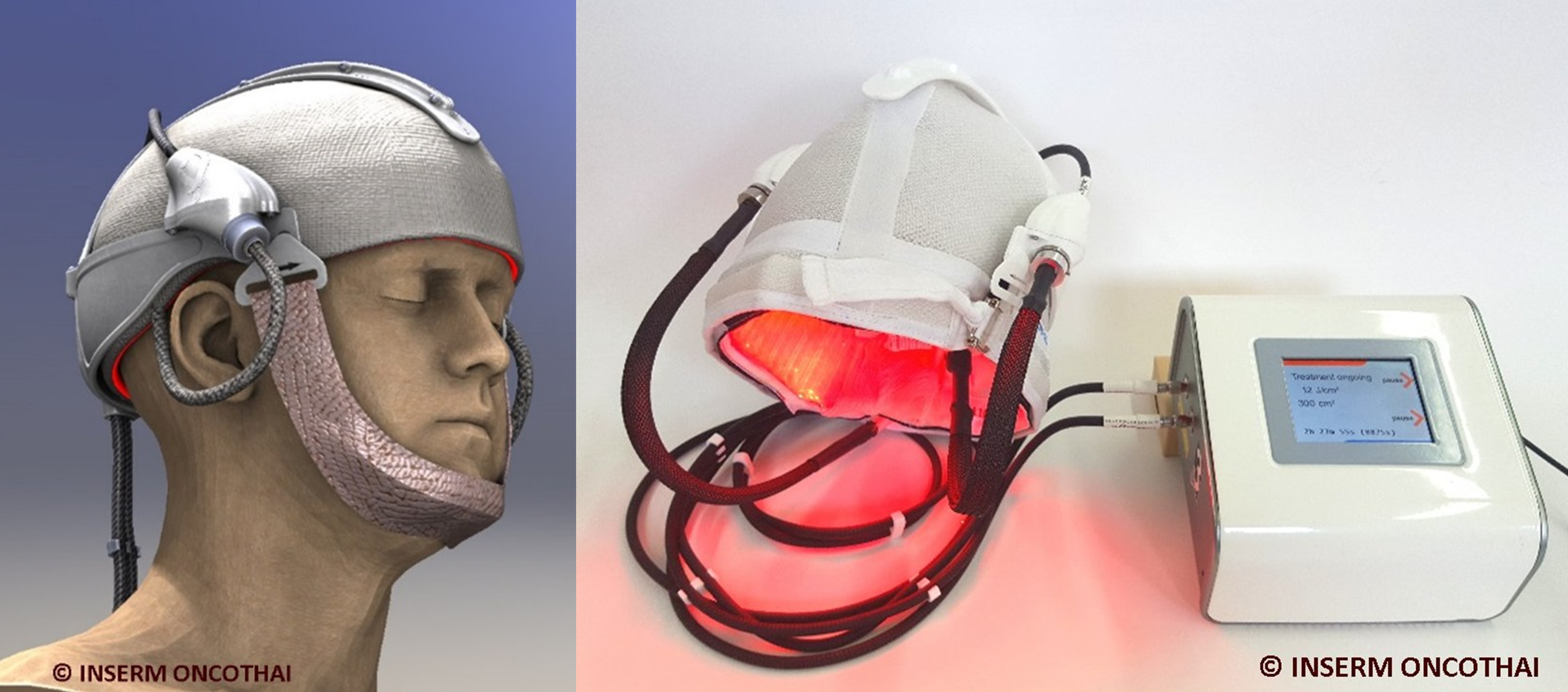
To improve PDT, a new light emitting device called PHOS-ISTOS®, composed of optical fibers, has been developed. This flexible device can provide a homogeneous light of low intensity, to provide a controlled and less painful treatment. The aim of the study is to evaluate the non-inferiority and the tolerance of the new PHOS-ISTOS® device, to treat actinic keratosis of the scalp and the forehead (PHOS-ISTOS®-PDT) compared to the MAL -PDT Conventional (C-PDT).
This is a prospective, comparative (intra-individual, split-face), randomized, open and bi-centric study of the non-inferiority of PHOS-ISTOS® PDT compared to C-PDT using the Aktilite® panel CL128 as a control device.
The AK will be diagnosed clinically, symmetrically distributed on the scalp and / or the forehead and will receive:
- In zone A: C-PDT treatment with an Aktilite® device
- On zone B: PDT treatment with the PHOS-ISTOS® device
Major patients with a clinical diagnosis of at least 10 untreated, unpigmented and non-hyperkeratotic AK lesions in the forehead and / or scalp will be included.
Patients will present themselves to one of two investigation centers for a treatment session (day 1) and will be followed at 7 days, 3 months and 6 months. A second treatment session may be performed at 3 months in cases where an incomplete response is observed.
Principal Investigator: Prof Laurent MORTIER
Co-investigator: Prof Rolf Markus Szeimies (KVEST)
Scientific coordinators: Prof Serge MORDON
Study Sponsor: Lille University Hospital
Primary endpoints: Show the non-inferiority in terms of the efficacy of PDT PHOS-ISTOS® compared to C-PDT at M3.
Secondary endpoints:
- evaluate treatment tolerance, including pain and local tolerance on D1 and D7
- evaluate the complete response rate to M3 and M6 for each treated lesion
- evaluate the cosmetic results at M3 and M6 (cosmetic aspect of the treated area)
- estimate the number of patients with 75% lesion reduction at M3 and M6
- evaluate quality of life and patient satisfaction (D7, M3, M6).
Evaluation criteria of the primary endpoint: To show the non-inferiority in terms of efficacy of the PHOS-ISTOS® PDT compared to the C-PDT at M3.
Evaluation criteria of the secondary endpoints:
- to evaluate the treatment tolerability including pain and local tolerance at D1 and D7
- to evaluate the complete response rate at M3 and M6 for each treated lesion
- to evaluate the cosmetic results at M3 and M6 (cosmetic aspect of the treated zone)
- to estimate the number of patients having a 75% lesion reduction at M3 and M6
- to evaluate the patient’s quality of life and satisfaction (D7, M3, M6).
Estimated enrollment: 42 to 47 patients
Clinicaltrials.gov identifier: NCT03076892
Contact and location:
LILLE : Pr Laurent MORTIER N° RPPS 10002307246
Service de dermatologie, hôpital Claude Huriez Rue Michel Polonowski, 59000 Lille
Tel: +33 (0)3 20 44 48 68
RECKLINGHAUSEN: Prof. Dr. med. Rolf-Markus SZEIMIES
KLINIKUM VEST GMBH
Knappschaftskrankenhaus Recklinghausen
Dorstener Str. 151, 45657 Recklinghausen Germany
Tel: +49 2361 56 3201
External links: Phos-Istos.com