Context and challenges
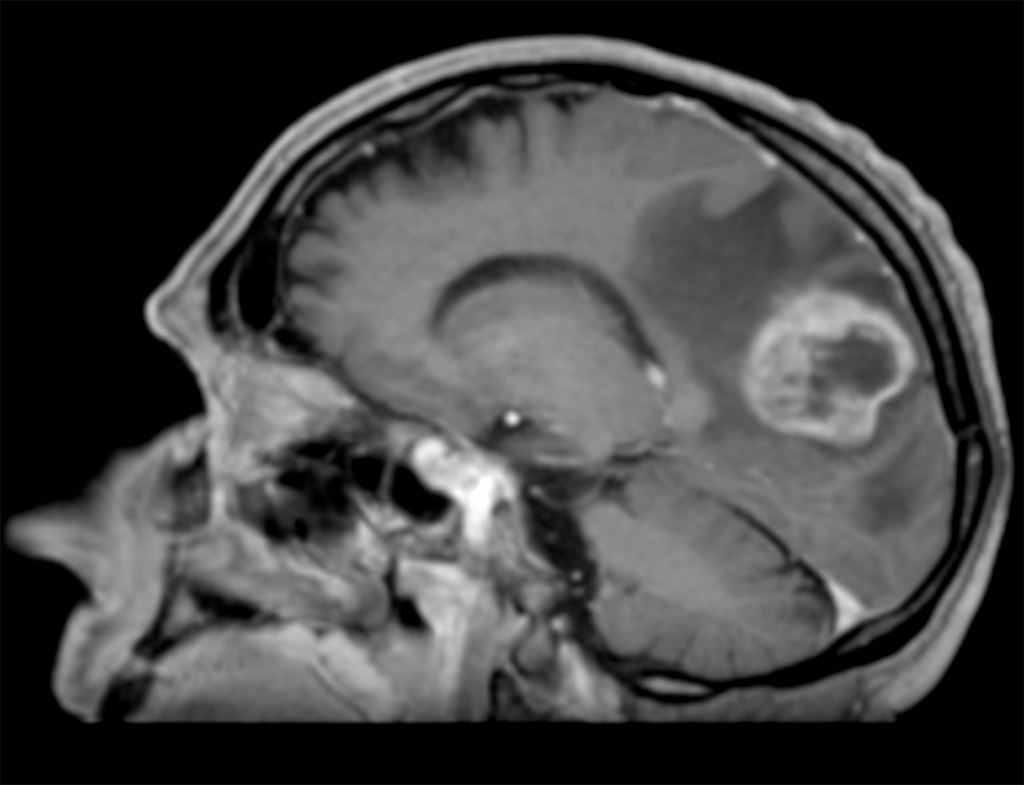
With a rising incidence up to 5 new cases each year per 100,000 inhabitants (13000 new cases per year in Europe), glioblastoma (GBM) is the third cause of death by cancer in young adults population (aged from 15 to 34). It is an incurable tumour whose median survival is less than 18 months. This oncology domain remains of great interest in both preclinical and clinical research. The standard treatment is based on surgical resection followed by brain irradiation associated with chemotherapy.
The goal of surgery is to achieve maximum reduction in tumour volume without affecting the functional outcome of patients according to their pejorative prognosis. Nevertheless, even when surgical resection is complete, the invasiveness of glioblastoma and its proliferating nature do not allow a local control with standard therapeutic protocols. Indeed, patients whose tumour location is not eligible for surgery have limited survival.
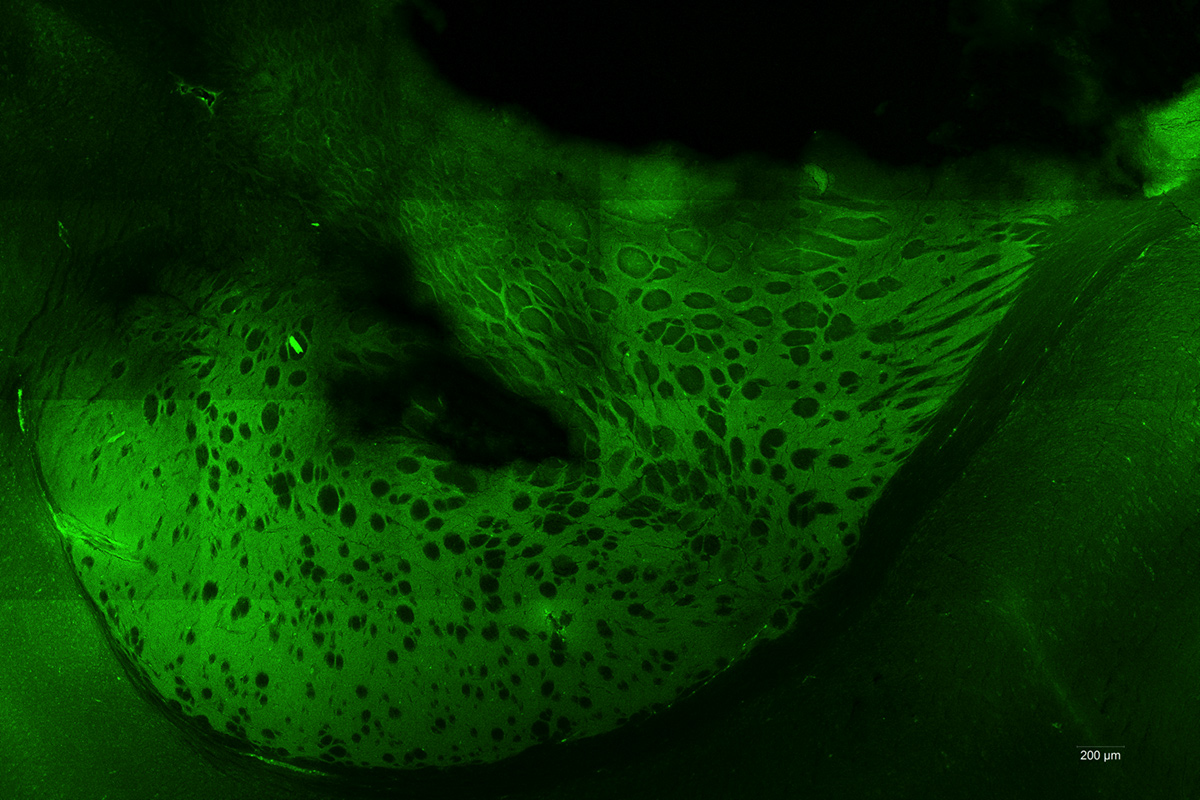
Photodynamic therapy (PDT) consists in exposing photosensitized tumour cells to laser light. Local or general administration of a pharmacological agent (precursor) is previously required to allow photosensitization of the cells. Our research focus on the 5-Aminolevulinic Acid (5-ALA), which induces a relatively specific photosensitizer accumulation: protoporphyrin IX (PPIX). The photosensitizer is present only in tumour cells and leads to their specific destruction upon illumination, while sparing healthy tissues.
The NeuroPDT research program developed by ONCOTHAI aims at developing an effective, reproducible and selective pattern for photodynamic therapy using 5-ALA. Nowadays, the on-going preclinical studies are confirming the efficiency of the 5-ALA laser therapy while revealing the harmlessness of its delivery. As a clinical approved molecule of 5-ALA for fluorescence guided resection, this therapy could be rapidly transferred to the clinical practice.
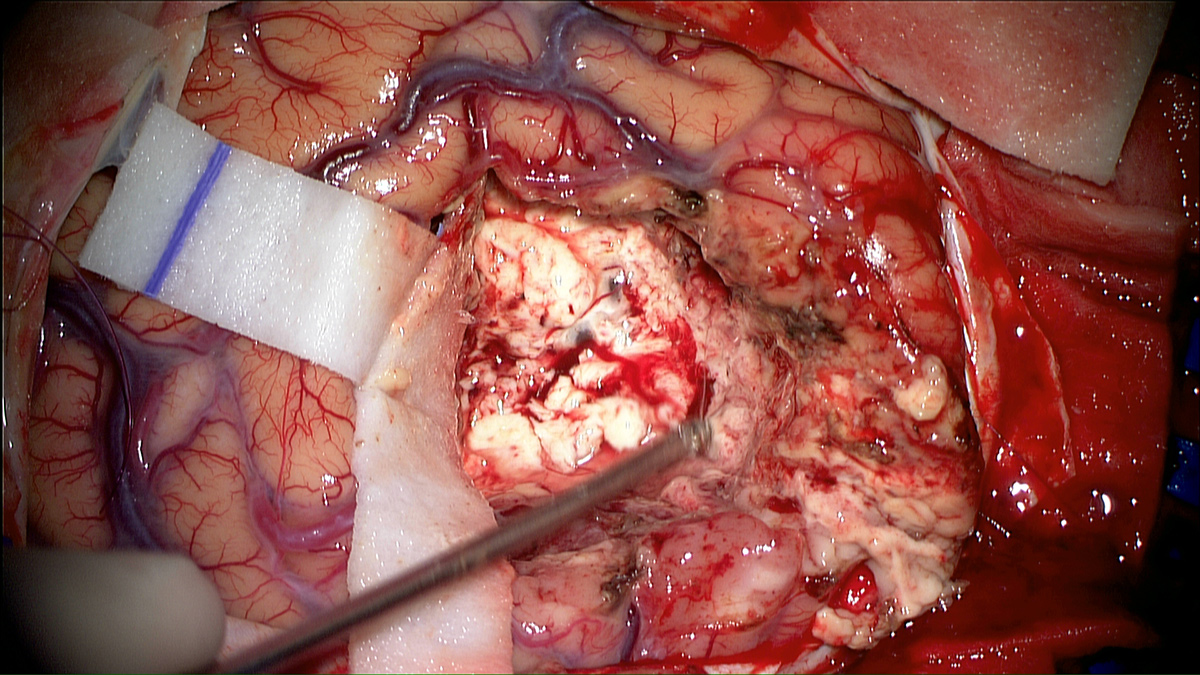
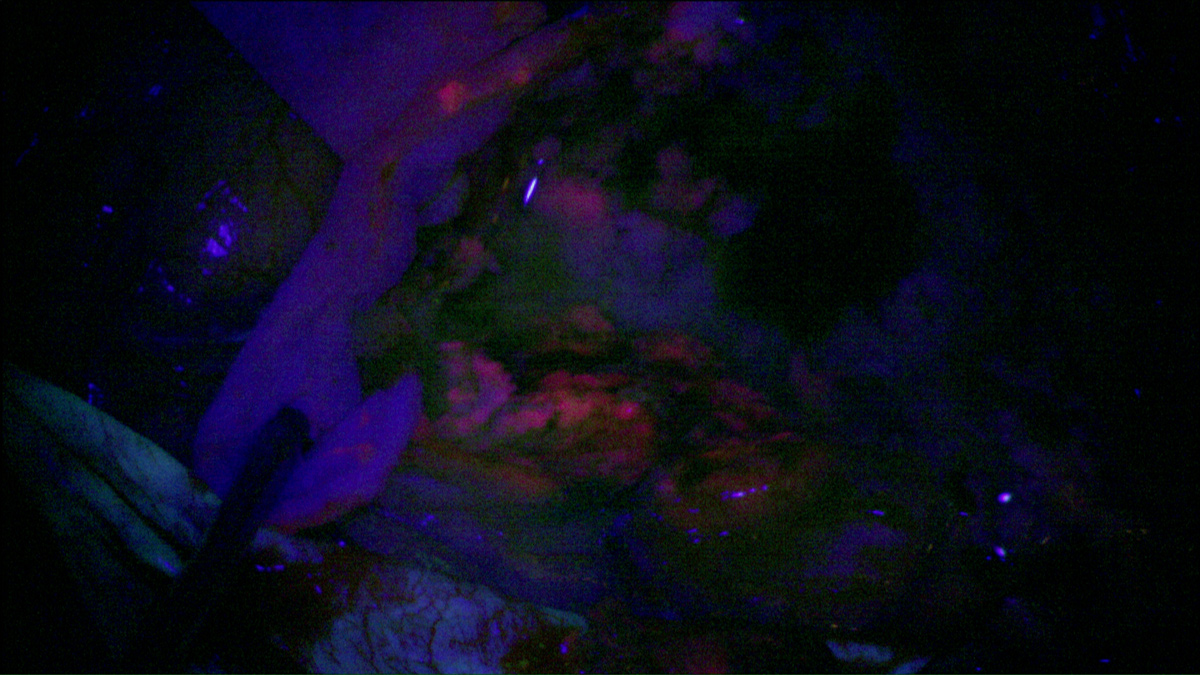
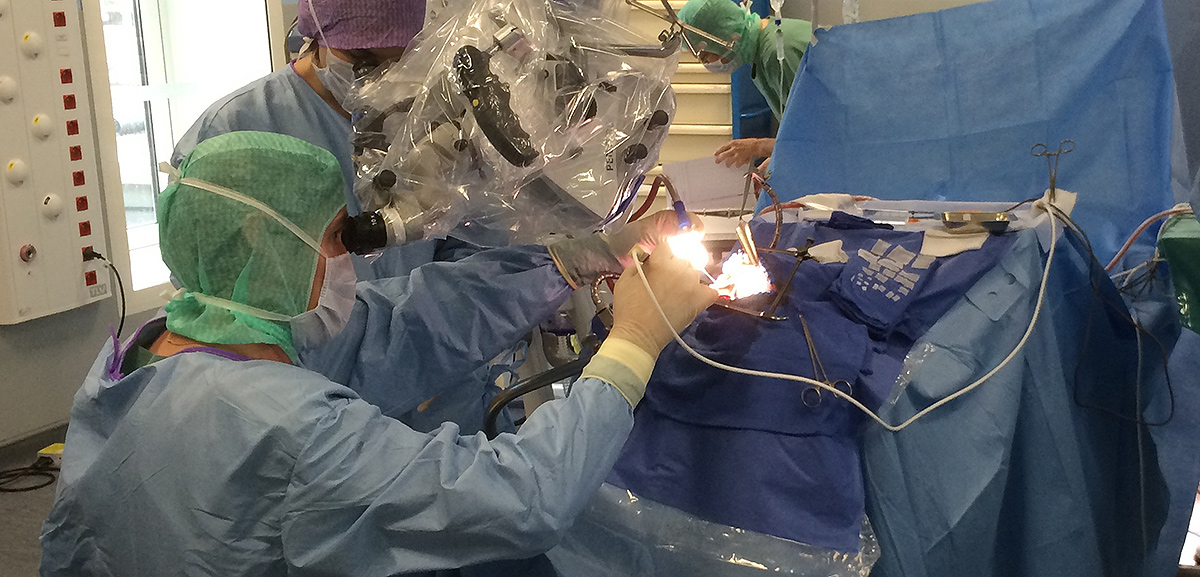
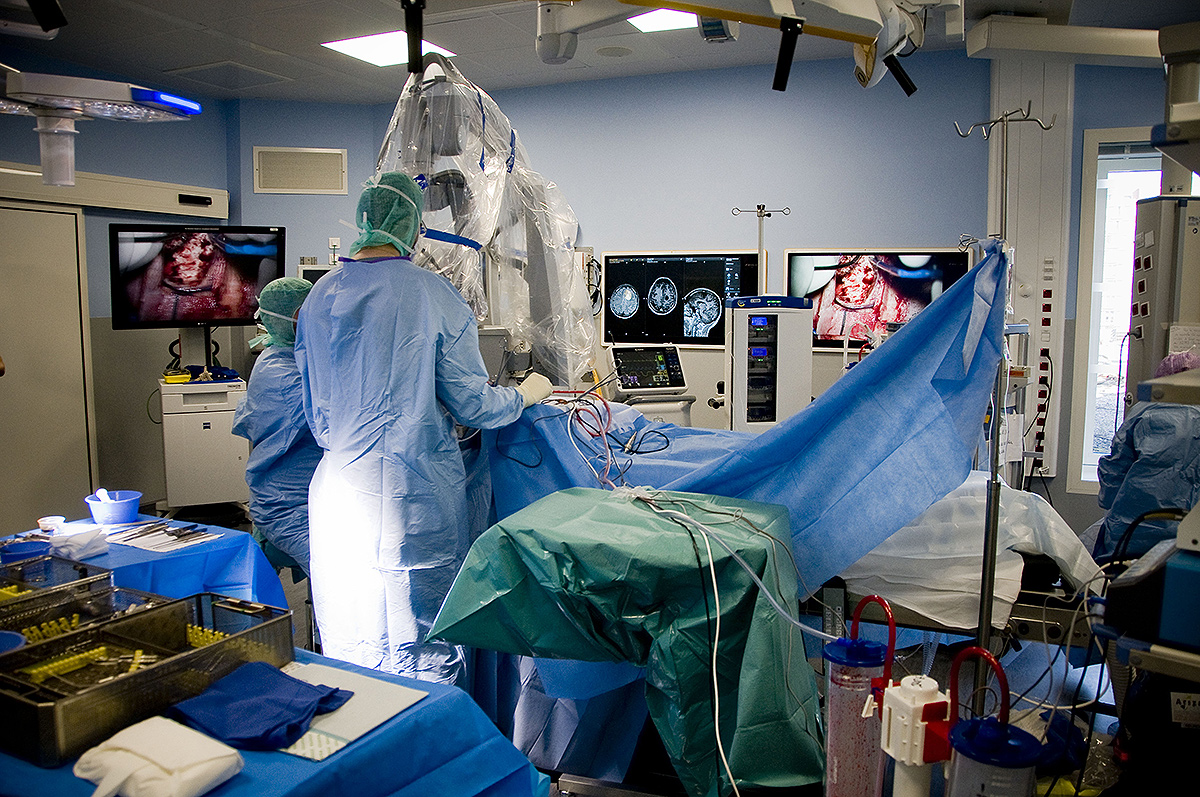
Using photodynamic therapy, the light exposure of photosensitized cells can be achieved according to two different patterns: exposure to red light of the operative site during surgery or insertion in the brain of optical fibers, for treatment distant from surgery. The latter solution could also represent an alternative therapeutic option for patients with inoperable or recurrent glioblastoma.
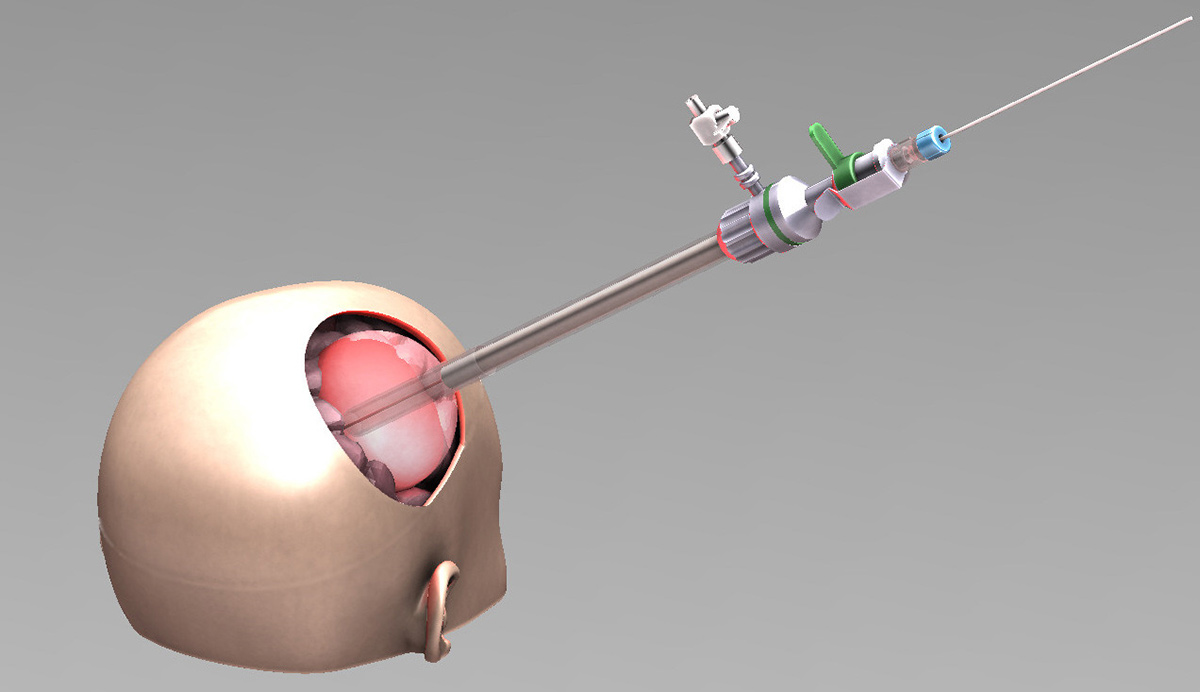
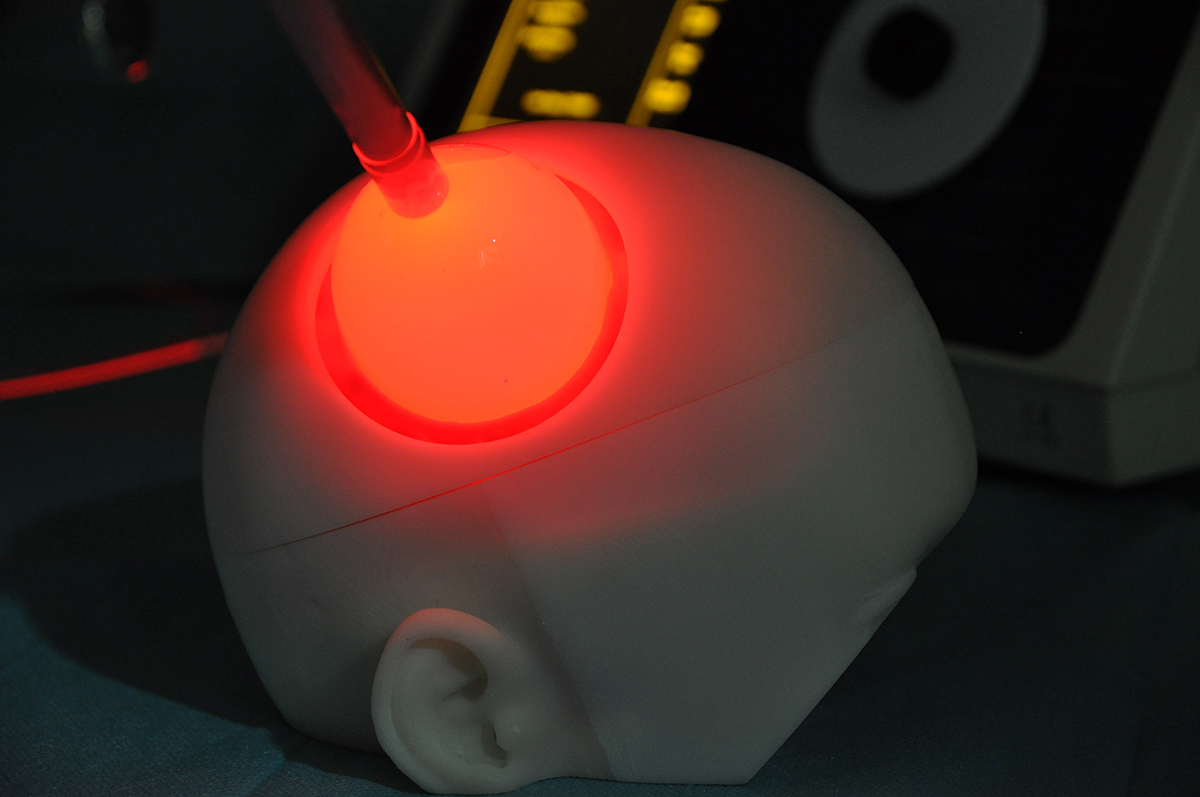
Eventually, the expected results would lead to a significant improvement in patients’ overall survival subsequently to a selective tumor reduction, assessed by MRI.
Goals
The purpose of the project is to propose two distinct treatments patterns: intraoperative photodynamic therapy (intraoperative PDT) and interstitial photodynamic therapy (iPDT). Indeed, these two modalities are complementary since intraoperative PDT is a promising adjuvant treatment to enhance the current therapeutic armamentarium (surgery, radiotherapy and chemotherapy) and iPDT is a new modality for the treatment of non-operable glioblastomas.
Integrating these new treatments in the management of the patient requires several research stages: preclinical study - clinical studies - methodological developments.
A pre-clinical study was conducted in the laboratory to validate the assumptions of efficacy and optimize the PDT treatment strategy.
Optical fibres coupled to a laser diode allowed a focused illumination of the tumour site in vivo. Thus, we were able to assess the response of glioblastoma treatment PDT. On another level, this study allowed us to optimize treatment modalities, controlled all the workflow steps (planning - treatment - monitoring) and finally validated the proof of concept of PDT.
The methodological findings concern both the image processing and treatment scheme for iPDT or intraoperative PDT.
Today, a comprehensive study of imaging techniques is implemented to identify the best solutions for planning, guiding and monitoring the treatment. The imaging panel covers both the metabolic imaging by Positron Emission Tomography (PET) and MRI.
In this context, PET images segmentation algorithms are developed to accurately quantify the metabolic activity of the tumor. This step is crucial because PET imaging combined with MRI images will ensure the definition of tumor invasion limits and help to adapt the treatment pattern, to get a better target for the therapy.
Where do we stand?
Clinical studies aim to validate all the implemented developments to deliver the optimal treatment.
Today, a single-center clinical trial is underway at the Lille University Hospital to demonstrate the feasibility and the safety of intraoperative PDT. This study, INDYGO (Intraoperative photodynamic therapy of glioblastoma) aims to deliver photodynamic therapy during surgery glioblastoma with a device developed in the laboratory.
On the international side, ONCO-THAI coordinates a European Network: SYNAPS (Synergizing Photodynamic Therapies for Neurosurgery). The network relies on a partnership between neurosurgical departments, research centres and health technologies companies. The objective of SYNAPS is to achieve a multicenter clinical trial and randomized across Europe.
Finally, this project will remove the constraints of using this technique for the treatment of these still incurable tumours. PDT laser treatment could quickly complete the therapeutic armamentarium for glioblastoma, and even become a new therapeutic option for inoperable tumour locations.




