Topic
Actinic keratoses (AKs) are erythematous and squamous skin lesions, rough to the touch, mainly present on sun-exposed areas. AKs are common; their prevalence in the U.S. and Europe is estimated between 11 and 25% of the population. In France, they concern 754/100.000 patients and represent the first cause of consultation in dermatology.
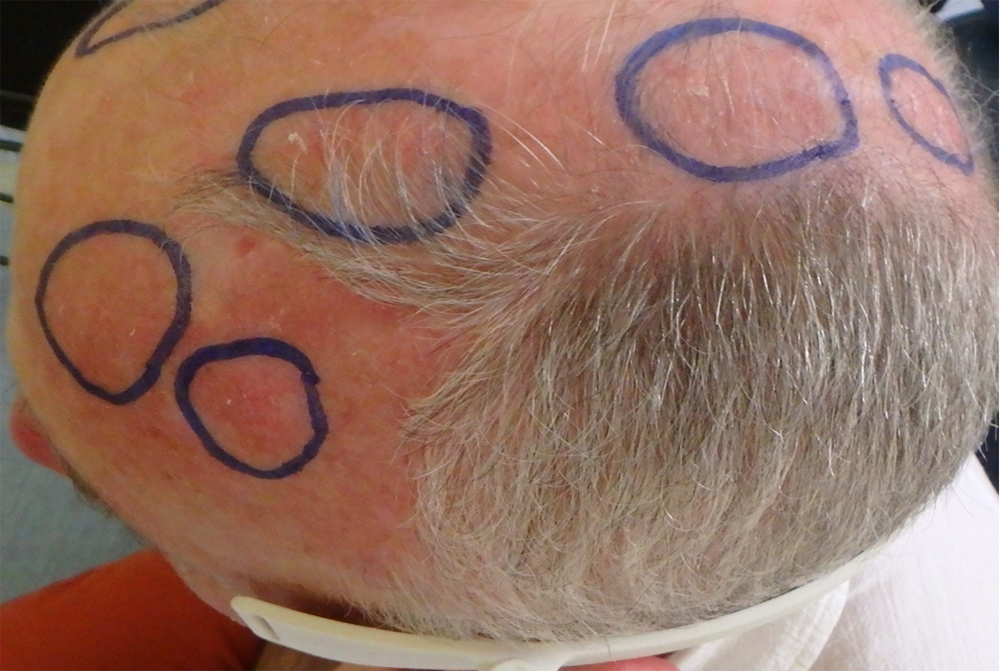
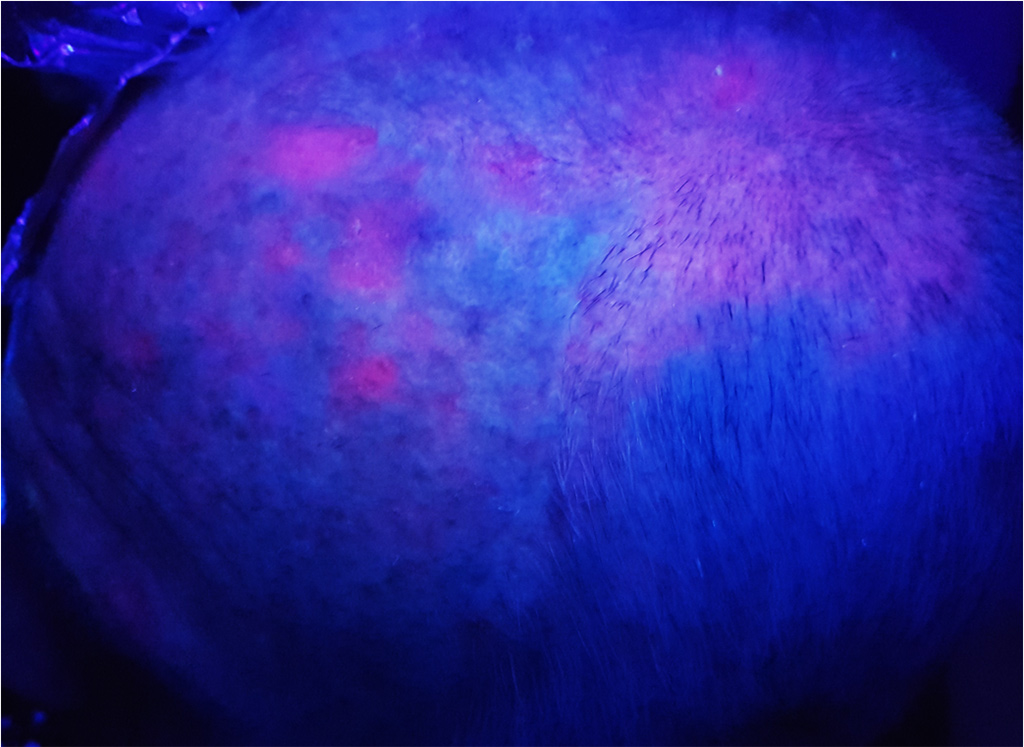
They are more likely to reach the elderly, with a clear phototype, who worked outside. Sometimes painful and hemorrhagic on contact, these lesions may evolve towards an invasive squamous cell carcinoma. AKs are often multiple. The apparently healthy skin between lesions presents abnormalities in histology and in biological analysis (leading to the notion of “field cancerization”).
It is estimated that for an individual with 7.7 AK, the probability that at least one of these lesions progresses to invasive squamous cell carcinoma over a period of 10 years is 10%. Malignant potential and the unpredictable evolution of AKs led to a consensus on the need to treat.
Photodynamic therapy (PDT) relies on the exposure to a light of a particular wavelength of photosensitized tumor cells. In dermatology, the photosensitization is given by the topical application of methyl aminolevulinate (MAL) contained in the cream Metvixia® (Galderma SA). Applied on the area to be treated, this pharmacological agent induces a specific accumulation of the photosensitizer Protoporphyrin IX (PpIX) in the tumor cells.
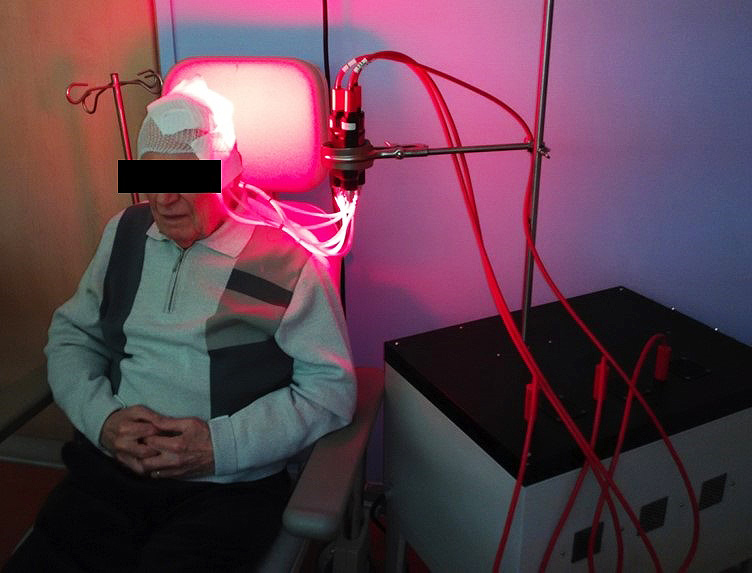
The light source most commonly used in Europe to activate the PpIX is the Aktilite CL 128 lamp (Galderma SA). This lamp, which is a rigid LEDs panel, emits a red light at a wavelength of 630 nm and delivers a light dose of 37J/cm² (75 mW / cm²).
Although often described as very painful by patients, PDT remains a first line treatment for the management of multiple AKs because it allows to treat large areas with a satisfactory response rate and excellent cosmetic results.
Goals
DermatoPDT program aims to develop new illumination devices and protocols, which should partially solve these limitations.
Gathering technical and software platforms, the program includes in particular research on the mathematical modeling1 of the interaction between light and biological tissue, and the development of softwares allowing the analysis and comparison of light sources2.
Illustration of the emission spectrum of a conventional illumination device for PDT in dermatology (blue), the normalized absorption spectrum of PpIX (green), and effective spectral irradiance (purple).
The DermatoPDT research program is also based on the development of new devices, integrating new illumination modalities3.
Within the framework of project ANR-12-EMMA-0018, the ONCO THAI unit contributed to the development of a new illumination device: Flexitheralight4.
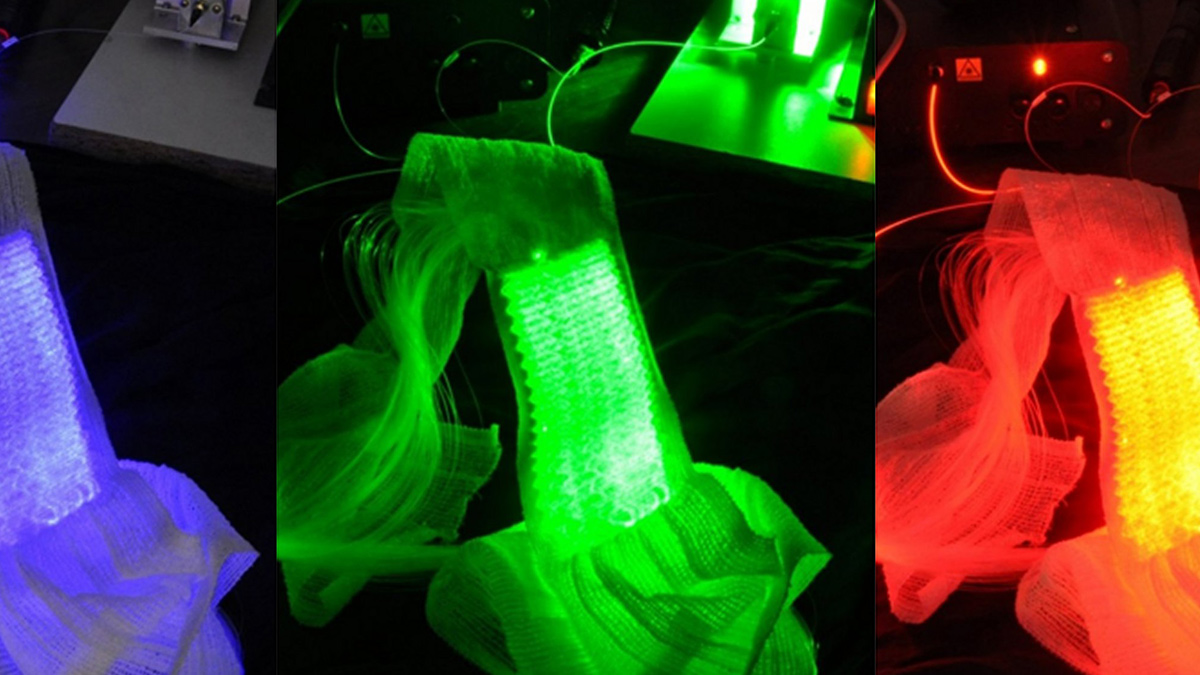
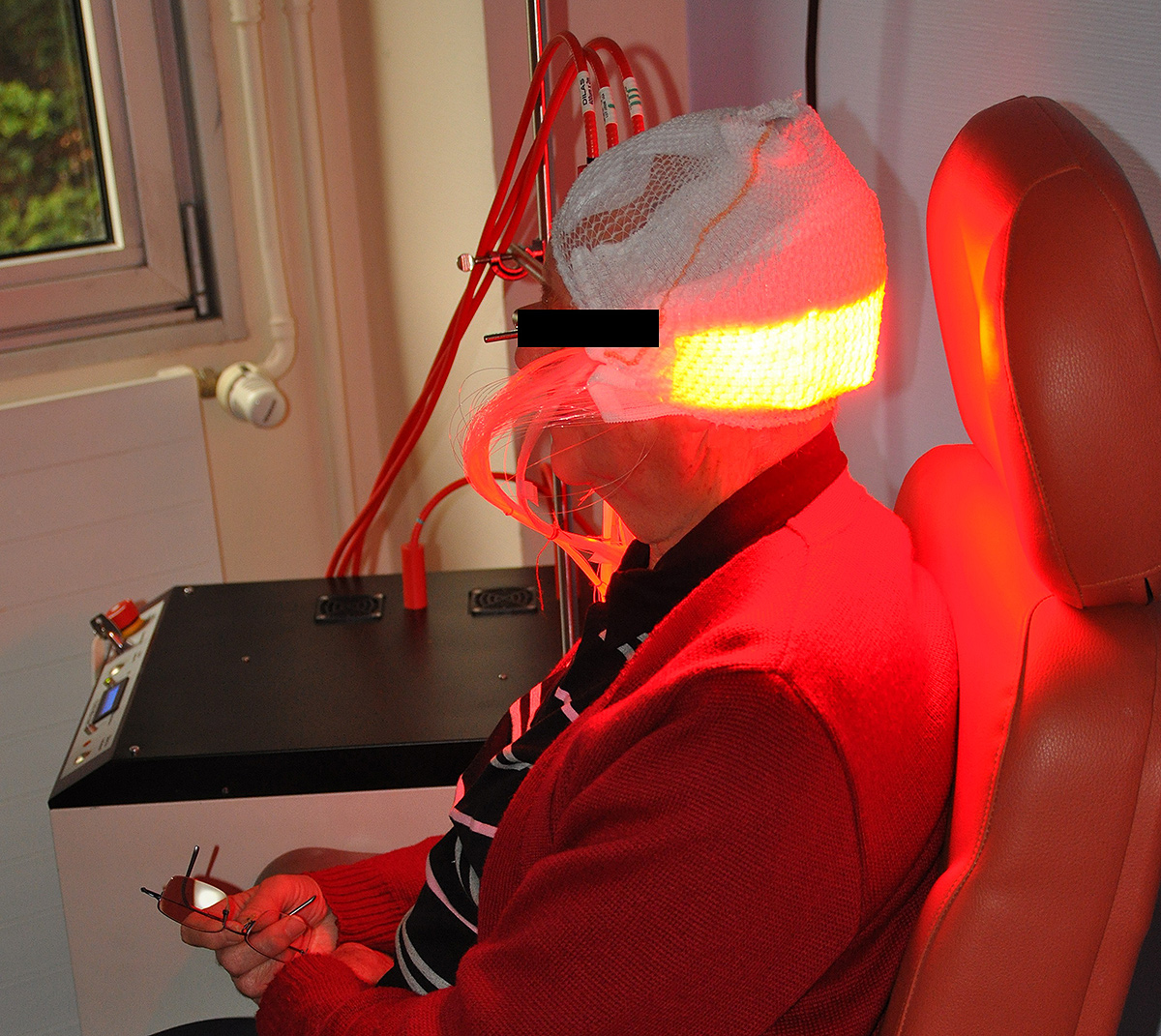
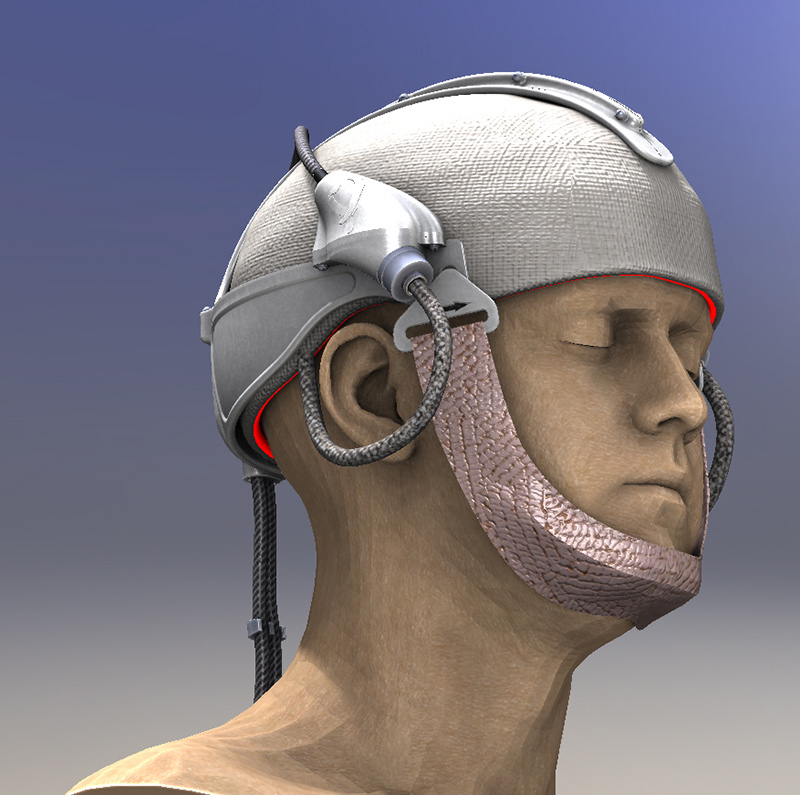
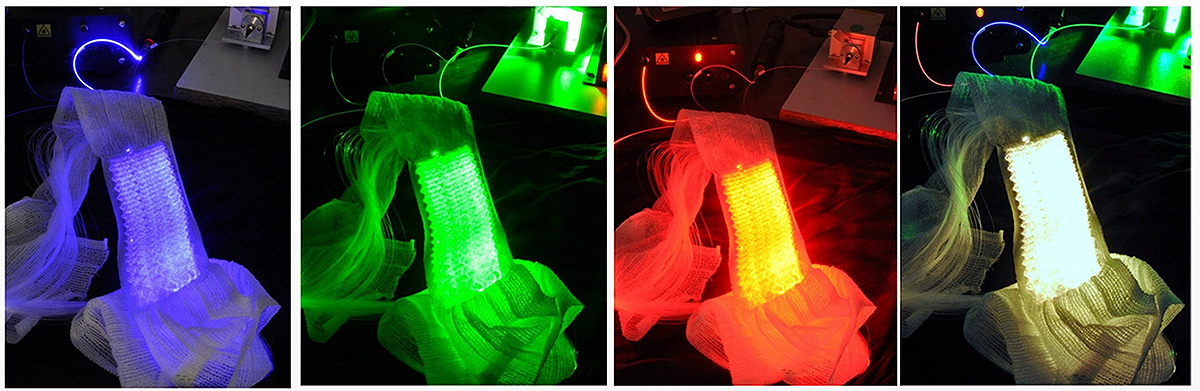
Based on the use of a light emitting fabric, conformable to the complexity of human anatomy, it guarantees a homogeneous illumination on all the lesions (especially on the scalp). In addition, light emitting fabric diffuses a red light (635 nm), with a low fluence rate (12 mW / cm²) in order to minimize the pain usually reported by patients.
In 2013, the ONCO THAI unit initiated the European project CIP 621103 Phosistos, and its partnership with various laboratories and manufacturers throughout Europe, resulted in the development of a new device integrating a mature light emitting fabric technology, more functional and industrializable5.
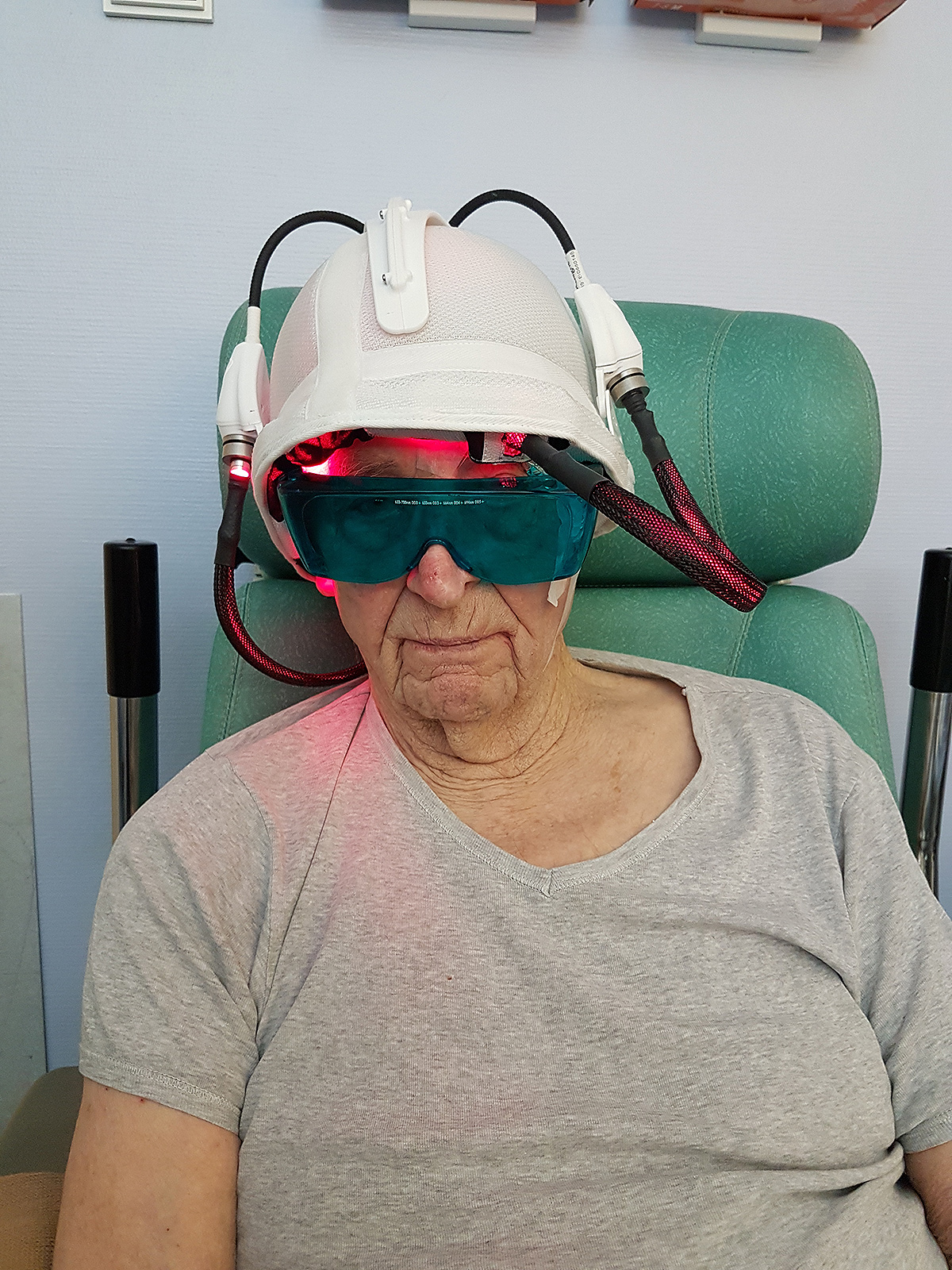
Where do we stand?
In 2016, Phosistos6 was evaluated in the management of AKs in a comparative, intra-individual, randomized phase II study conducted in the dermatology departments of Lille University Hospital in France, and Klinikum Vest in Germany. The main hypothesis of this study was the safety of both Phosistos device and illumination modality, the non-inferiority in terms of efficiency and the better tolerance compared to conventional PDT.
About forty patients with at least 10 AKs were included in this study. The included lesions were divided into 2 groups: the first group received the conventional PDT (with the rigid panels and a high fluence rate: 75 mW/cm²) while the second group was treated with the Phosistos device. The 3-month therapeutic response and clinical tolerance were evaluated for both methods and then compared.
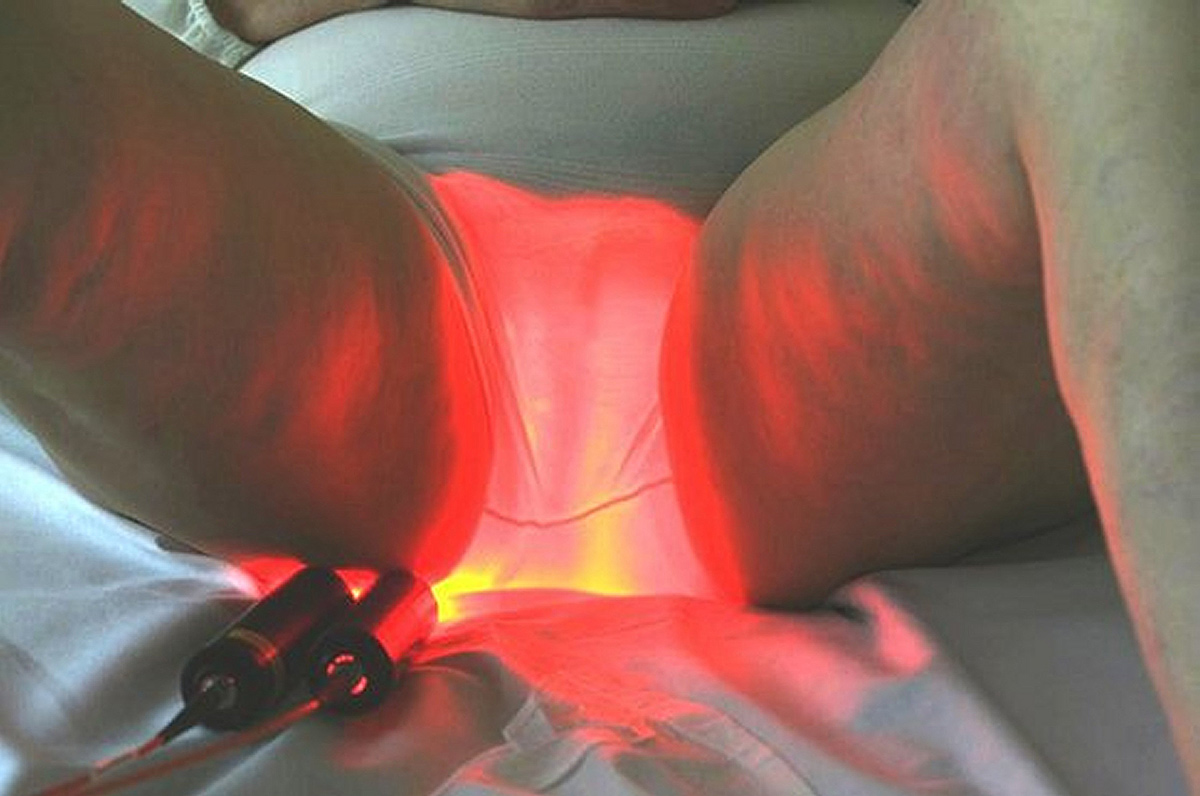
The results show an efficacy of the device Phosistos comparable to the conventional PDT, with practically no pain.
Other applications of these devices are being investigated, such as Paget Extra-Mammary, or Folliculitis decalvans.
The light emitting fabrics technology can be associated with different light sources and can deliver different illumination modalities, in compliance with regulatory constraints (such as the Levulan® in the US, which requires illumination at 417 nm) and clinical constraints.
1 Vignion-Dewalle, A.S., et al., A software for analyzing and comparing the light sources available for PDT in dermatology. EuroPDT Munich, 2017.
2 Vignion-Dewalle, A.S., et al., Comparison of three light doses in the photodynamic treatment of actinic keratosis using mathematical modeling. J Biomed Opt, 2015. 20(5): p. 58001.
3 Mordon, S., et al., Light emitting fabric technologies for photodynamic therapy. Photodiagnosis Photodyn Ther, 2015. 12(1): p. 1-8
4 Phase II study evaluating the non-inferiority of the Flexitheralight device compared to conventional phototherapy (PDT): n° 2013-A01096-39 – ID clinical trial: NCT03076918
5 (www.phosistos.com)
6 Phase II study evaluating the non-inferiority of the Phosistos device compared to conventional phototherapy (PDT): n° 2016-A00010-51 – ID clinical trial: NCT03076892