Topic
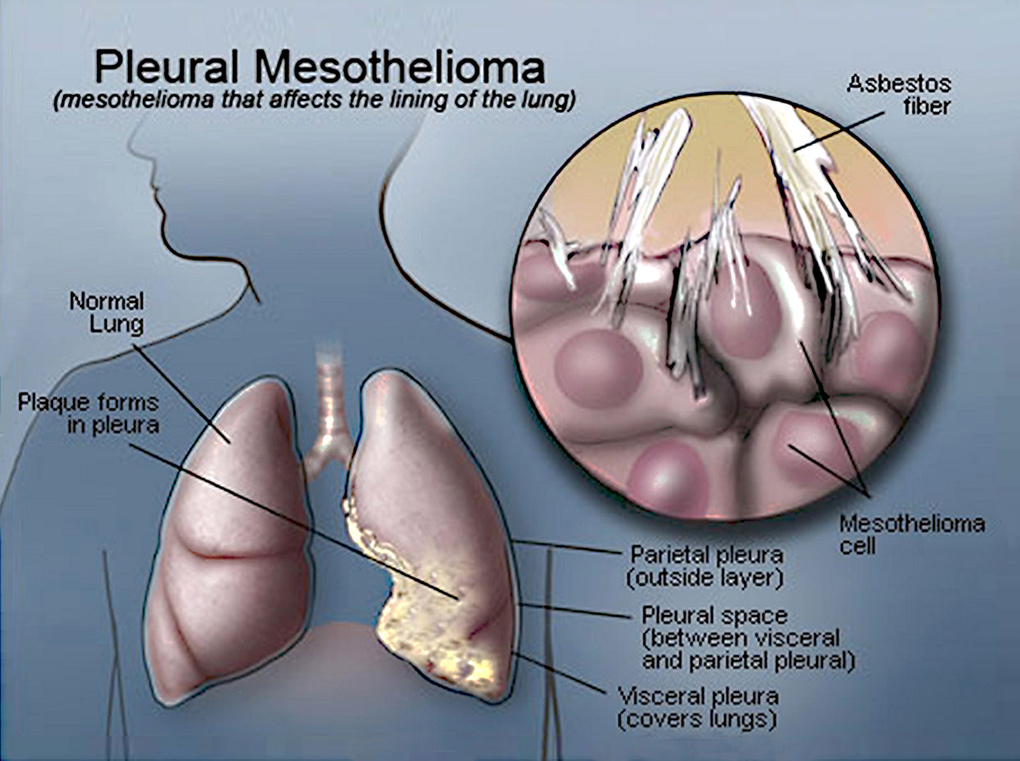
Malignant pleural mesothelioma (MPM) is an aggressive serosal tumor of the pleura. Its main aetiological agent is an exposure to asbestos fibers, mostly work-related, and the disease appears after a latency of 30 to 40 years after initial exposure. MPM is considered as a rare tumor: in France, its incidence is 900 cases every year. However, its incidence is rising throughout the world because of the increasing use of asbestos until the 1970’s and will peak in the next decade. We also fear a pandemic rise of MPM in the future with developing countries (China, Brazil, India…) still using asbestos today. MPM has a poor prognosis with a median survival of less than a year. This can be explained by the delay of diagnosis due to late symptoms, with a disease already advanced locally, the difficulty of obtaining a confident anatomopathological diagnosis, and a complex treatment with deceiving outcomes.
Treating MPM remains a challenge. MPM shows a strong resistance to chemotherapy, which is often the unique treatment available for patients with advanced and unresectable disease, or who cannot sustain a heavy thoracic surgery, with a median survival of 12,1 months. Surgery offers the best chance of survival for this still incurable disease. However, after the most complete tumor resection, microscopic tumor cells persist and surgery should be associated with a local adjuvant treatment. Up to this day, there are no international recommendations on surgical treatments with a curative intent. Surgery is still investigational and should be part of a multimodality treatment and included in a prospective clinical trial. In January 2012, a clinical French network of regional expert centres for the care of MPM was created: “MESOCLIN”. It is financed by the National Institute against Cancer or INCa. This network is coordinated par the expert centre of Lille, in close cooperation with “Mesopath”, a French anatomopathological expert network.
Medical interest for this cancer has evolved during these last years, with a real investment in its treatment: with the notion of reasonable surgery, with relatively efficient cyto-toxic drug combination, with the emergence of biotherapy and new radiotherapy modalities, and especially the development of concerted multidisciplinary strategies which allow to build randomised, prospective clinical trials, that had been lacking on this field. The recruitment of patients with MPM is important in Lille, due to strong professional and environmental exposure to asbestos fibres in the “the Hauts de France” area: shipyards, metallurgy, building industry…
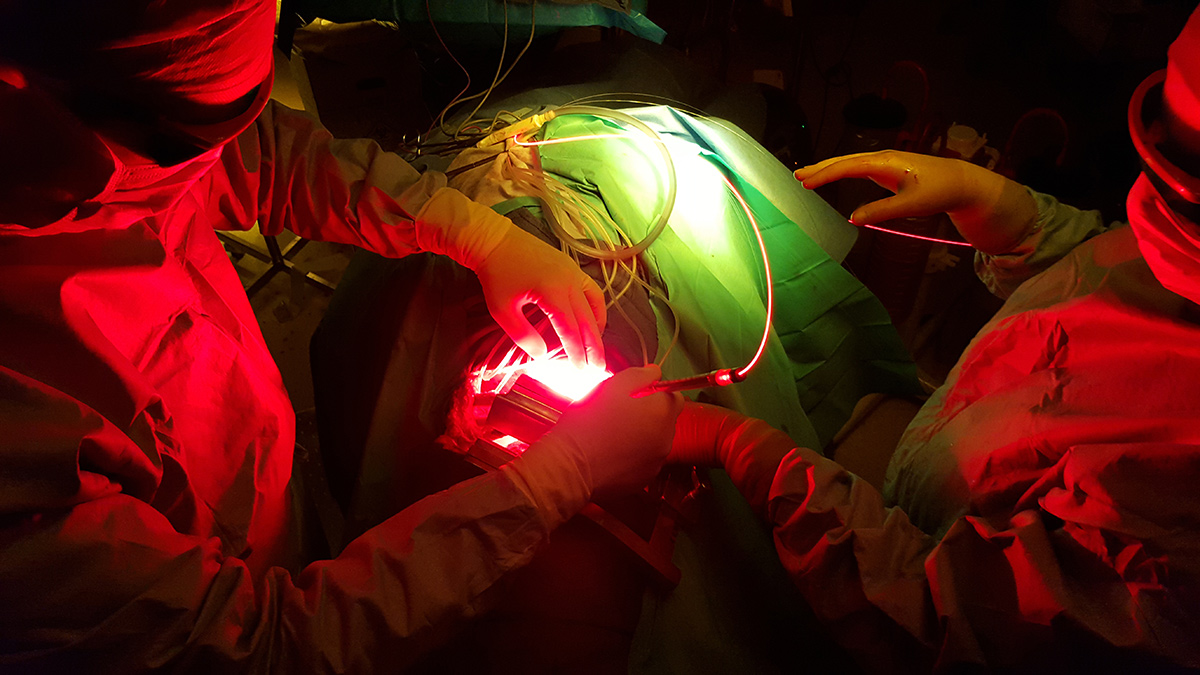
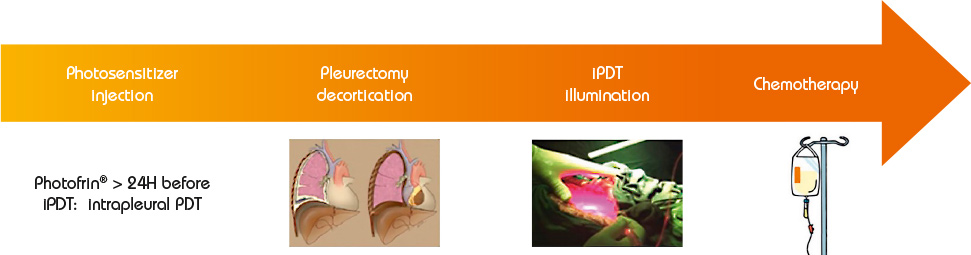
Photodynamic therapy is a treatment of ancient principle, but is relatively new in the multimodal management of MPM. The effect of PDT requires the interaction of 3 components: a photosensitizer (PS), oxygen in the tissue and light with the specific wavelength activating the PS. None of them are individually toxic but when put together they induce a tumoricidal photochemical reaction. When tumour cells absorb the light, the photosensitizer produces free radicals and oxidation reactions which lead to the death of the treated cells and kill tumour angiogenesis. Intrapleural PDT is based on a two-stage process: intravenous administration of a photosensitizer with a specified drug dose and drug-light interval before surgery, and intraoperative illumination of the pleural cavity, after maximal resection of the tumor, by a laser source at an appropriate wavelength, and at a specified light dose. The appeal of PDT as an adjuvant treatment relies on its relative tumor selectivity, depending on a photosensitizer able to direct itself and remain longer in the tumor cells than in the normal cells, and an illumination restricted to the cancer superficial zone.
For a few decades, photodynamic therapy has been the subject of many studies (especially phase I and II studies) as part of a multimodal treatment of malignant pleural mesothelioma, including extra pleural pneumonectomy (EPP) or pleurectomy decortication (P/D). If preliminary results were disappointing, two recent studies by Friedberg (USA) have demonstrated that Photofrin®-mediated PDT, after complete macroscopic resection, and associated with cisplatin based chemotherapy, offers a remarkable survival with reasonable toxicity. Even in patients with locally advanced stages of MPM, and when a lung-sparing procedure (P/D) is performed, survival was much higher than without adjuvant PDT: the median OS (Overall Survival) was 31,7 months (95% [CI] 9-54,3 months).
Goals
INSERM U1189 laboratory, in collaboration with the departments of Pulmonology and Thoracic Oncology, and Thoracic Surgery, in Lille University Hospital, have started an ambitious project aiming at developing intrapleural PDT for MPM.
On the clinical side, the objective was to combine intrapleural PDT to P/D and adjuvant chemotherapy, for patients with MPM. There was already a close connection between the two teams of France and USA, who met many times. The idea was to reproduce identically the surgical procedure and the PDT performed by the team of Philadelphia, thanks to their collaboration.
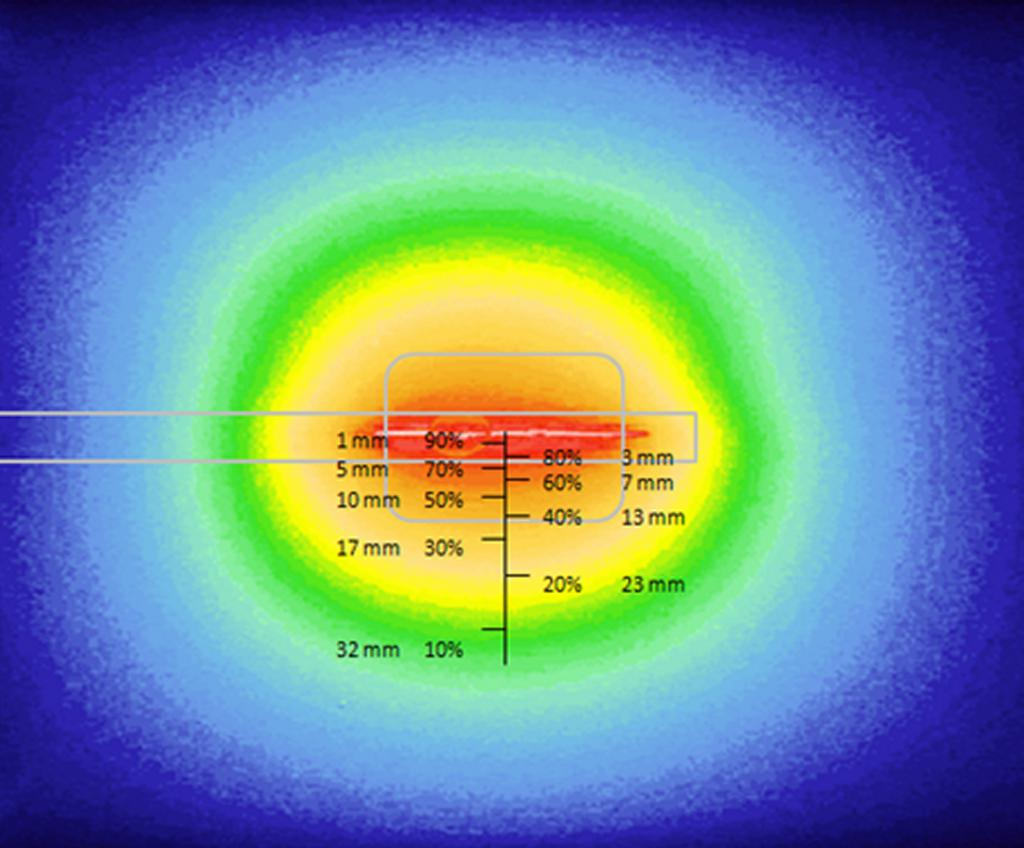
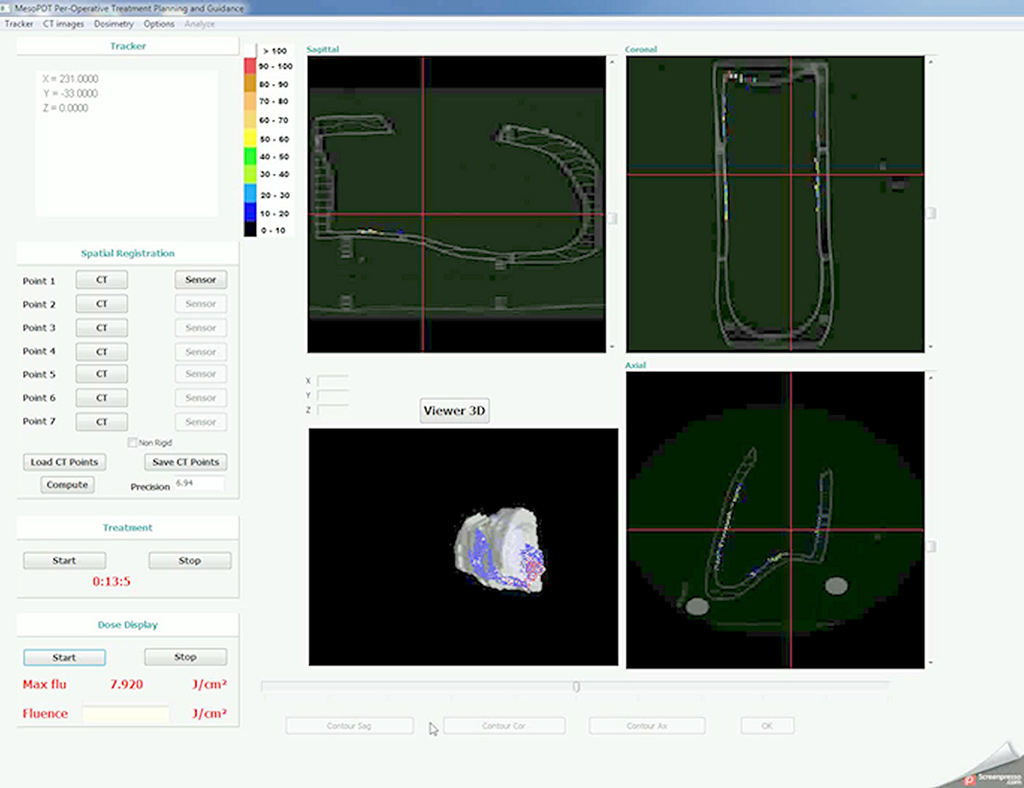
On the research side, the objective was to optimize the illumination through the light device and the development of an alternative intra operative dosimetry method for intrapleural PDT. Complete and uniform illumination of the pleural cavity is very difficult to obtain, due to its complex geometry and the presence of surrounding « noble » organs. The actual technique consists in filling the cavity with diffusing solution and moving the light device around the chest cavity. Today, light dosimetry is achieved using seven sensors collecting light dose and fixed inside the pleural cavity at strategic locations, but it does not give information about the light delivered between these points. The research progamme of INSERM U1189 is based on skills, already used for other pathologies: laser action modelling and medical imaging. The aim is to optimize light dosimetry and ensure complete and uniform light delivery to the targets, which is the key to the success of PDT.
Where do we stand?
With the support of the Regional Board of Nord Pas de Calais ans patients associations, a local phase II clinical trial is today ongoing at the University Hospital of Lille. Then, a multicentric randomized phase II trial will take place in France, to validate the benefit of PDT for MPM. This second clinical trial takes place within a Clinical Research Hospital Programme (PHRC) selected by the National Cancer Institute (INCa).
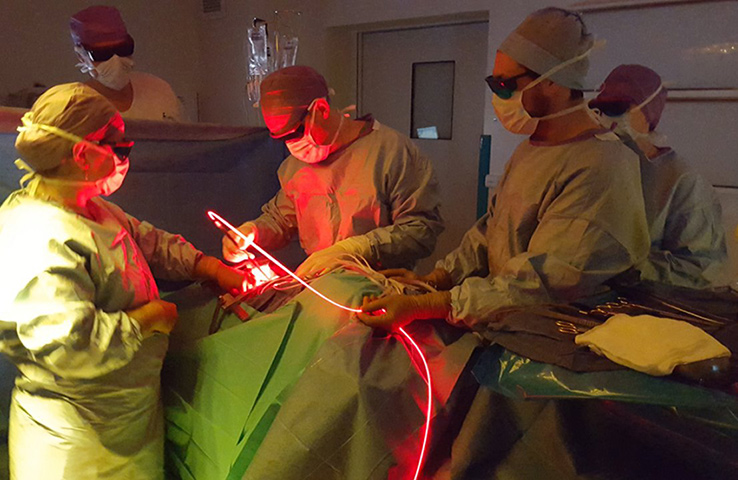
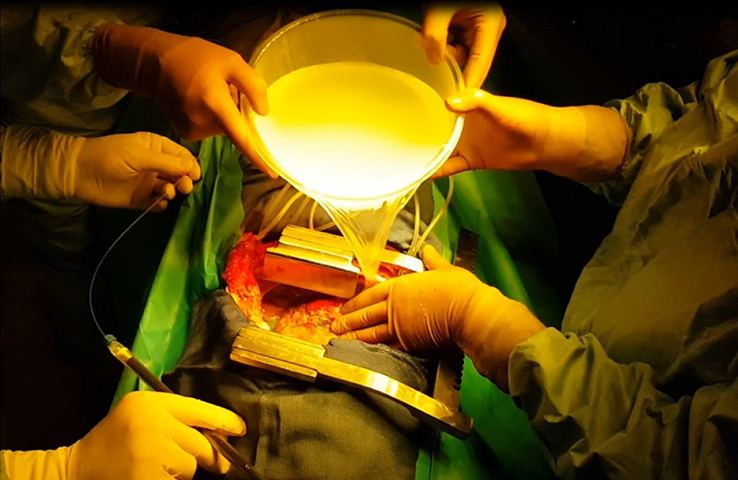
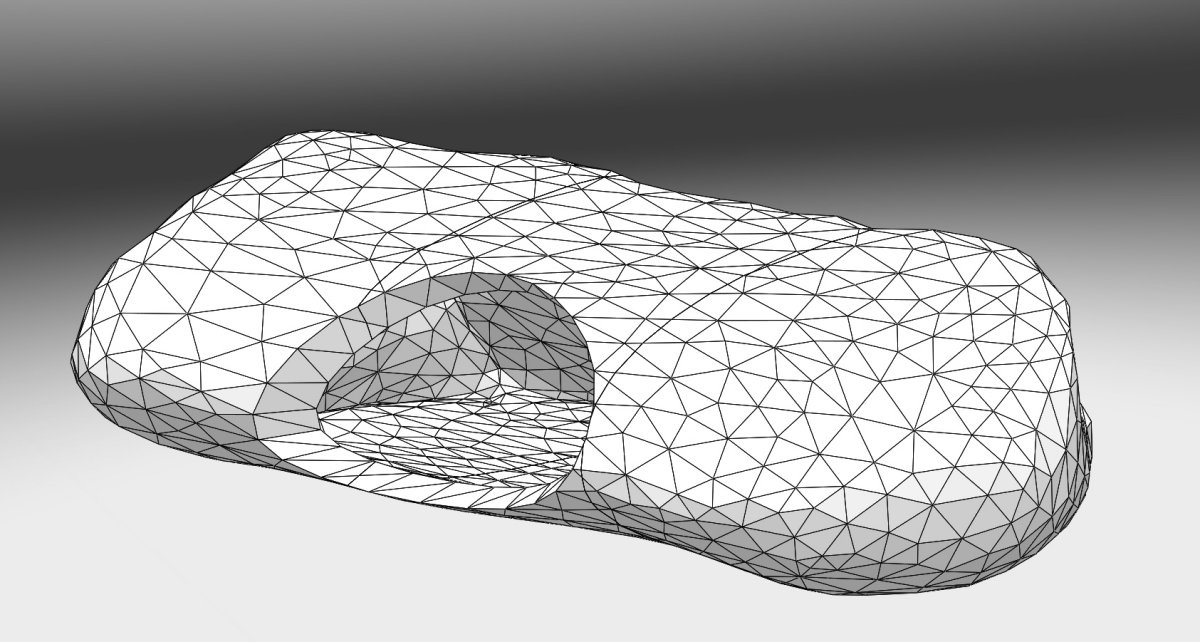
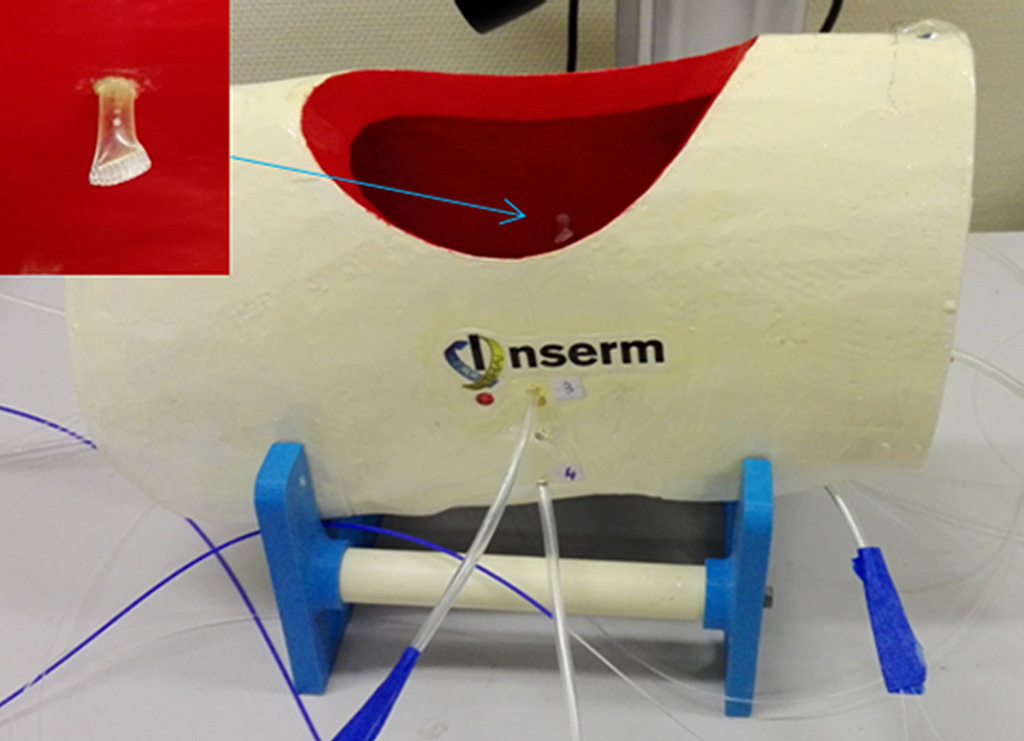
On the experimental side, an illumination profile of a light device has been defined and combined with an electromagnetic spatial tracking system, with 3D imaging (chest CT scan) visualization of of the light dose delivered in real time. This new dosimetry approach has been validated on a intraoperative hemithorax phantom, conceived and 3D printed within the laboratory.
Another path of research on which INSERM U1189 is working is the use of flexible light textile which could well be applied on the pleural cavity walls. Recent work, has demonstrated that flexible light textile could ensure homogenous light delivery on a peritoneal carcinomatosis animal model.