Intrapleural Photodynamic Therapy in a Multimodal Treatment for Patients With Malignant Pleural Mesothelioma
Ongoing / closed: closed
Research type (rétro/prospective): prospective
Synopsis:
Malignant pleural mesothelioma (MPM) is an aggressive tumour with poor prognosis (median survival <13 months), and high resistance to chemotherapy. Extended pleurectomy/decortication (eP/D) is a debulking surgery of MPM but cannot be considered as a curative treatment. Therefore it has been suggested that eP/D may be of interest if combined with intra-operative treatment and adjuvant therapies.
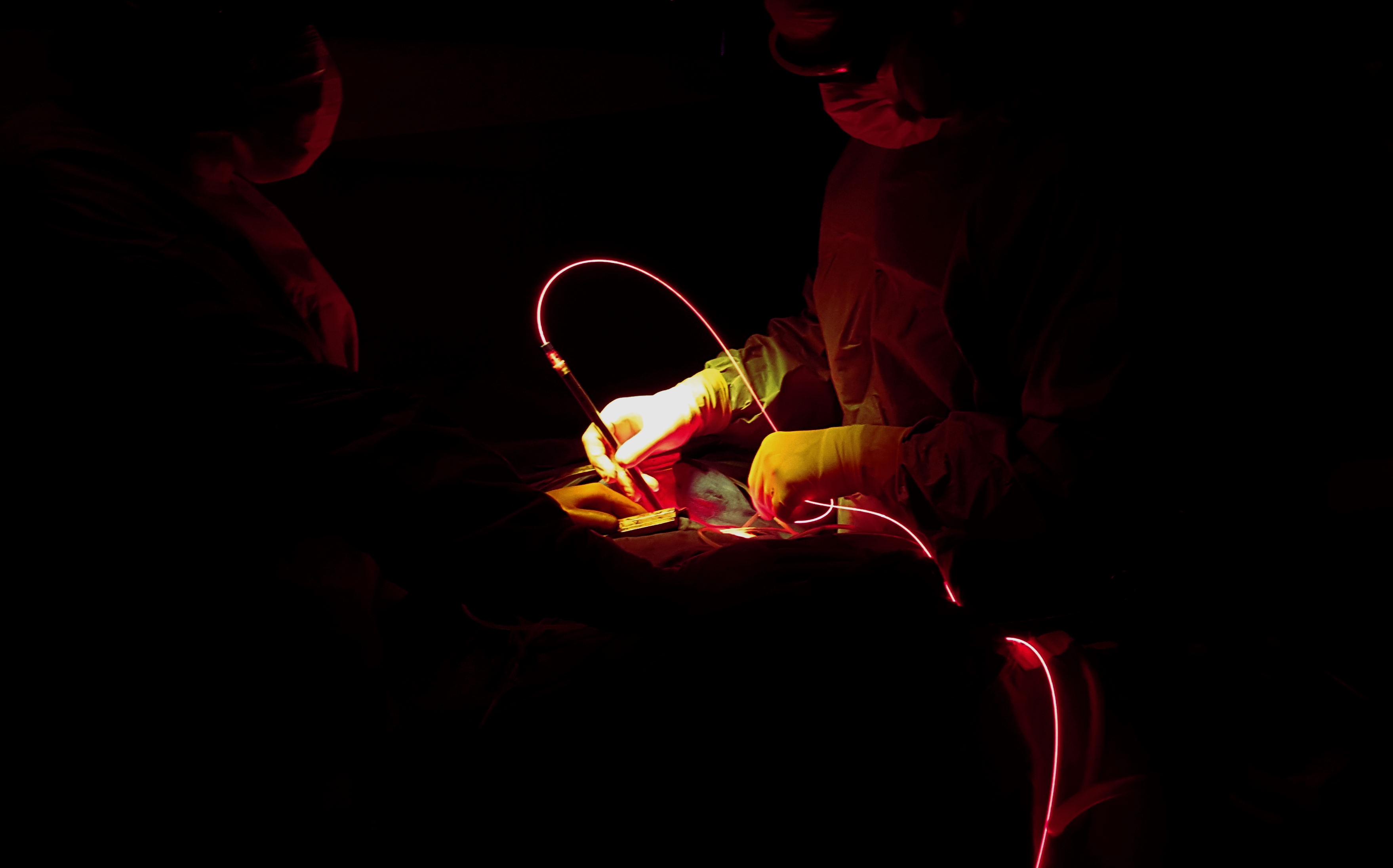
Photodynamic Therapy (PDT) is an innovative treatment based on the rationale that tumour cells, if previously treated with photosensitizing drugs (Photofrin), will die when exposed to light at a particular wavelength. Interestingly PDT might also stimulate anti-tumour immune response through the release of tumour antigens and induced inflammation.
PDT was tested in phase I-II trials for MPM in combination with EPP or eP/D, and chemotherapy. US studies from J Friedberg et al found very promising survival results in MPM when combining eP/D, but not EPP, intra-operative PDT and chemotherapy (cisplatin-pemetrexed), with a median overall survival of 31.7 months.
However, the definitive value of intra-pleural PDT combined to eP/D in the treatment of MPM still need to be validated. The same multimodal treatment has been established in Lille, the French national expert centre for MPM, with the help of our american colleagues. Therefore, this phase II trial proposes to patients to benefit from the combination of eP/D, intra-operative PDT then chemotherapy by cisplatin-pemetrexed and prophylactic radiotherapy.
Primary endpoint is the feasibility for the patients to have the full multimodal treatment of MPM including intrapleural PDT without unacceptable or unexpected grade III-IV toxicities. Secondary endpoints are PFS, OS, ORR, and quality of life. If the feasibility of such treatment would be confirmed in France, a multicentric, randomized trial comparing this experimental treatment vs control arm (same multimodal treatment without PDT) is planned.
Principal investigator: Pr. Arnaud SCHERPEREEL
Scientific coordinators: Pr. Serge MORDON
Study Sponsor: University Hospital of Lille - France
Primary endpoints: Number of Patients having the full multimodal treatment without unacceptable and unexpected toxicities (grade ≥ 3) graded According to NCI CTC Version 4.0 [ Time Frame: at 12 months ]
Secondary endpoints:
- Number of responders or stable patients after surgery [ Time Frame: at 12 months ]
- Progression-free survival (PFS) [ Time Frame: through study completion, an average of 13 months ]
- Quality of life assessed by a specific questionnaire MPM (HCCA EORTC-30) [ Time Frame: Baseline and at 12 months ]
Evaluation criteria of the primary endpoint:
Proportion of patients receiving complete multimodal treatment, including intrapleural PDT (expected target: 66% minimum, i.e. 4/6 patients), according to the following criteria:
- Realization of the complete multimodal treatment: pre-operative 24h or 48h injection of the photosensitizer (Photofrin), eP/D type resection surgery, intrapleural PDT, prophylactic radiotherapy (21 Gy) of the drain holes and surgical scars, adjuvant chemotherapy by 4 to 6 platinum and pemetrexed cycles.
- Realization of this complete therapeutic protocol within the given time: at most within 9 months after surgery by eP/D (+ intrapleural PDT): prophylactic radiotherapy within 2 months postoperative and adjuvant chemotherapy (cycles of 21 days minimum ( maximum every 5 weeks) x 4 to 6 cycles) to begin within 3 months postoperatively.
- Evaluation of the safety profile: unacceptable and unexpected toxicities (grade ≥ 3) according to the National Cancer Institute (NCI) toxicity criteria (reviewed by an Independent Surveillance Committee).
Evaluation criteria of the secondary endpoints:
- Number of patients (responders or stable according to modified RECIST criteria) at 12 months postoperatively
- Progression-free survival (PFS) from date of inclusion to date of relapse
- Quality of life assessed by a specific MPM questionnaire (EORTC LCSS-30) pre- and post-therapeutic
- Response to treatment with tumor biomarkers and CT-coupled positron emission tomography (PET-CT)
Estimated enrollment: 6 patients
Clinicaltrials.gov identifier: NCT02662504
Contact and location:
Pr. Arnaud SCHERPEREEL
Pneumologie et Oncologie Thoracique, Hôpital Calmette, CHRU de Lille
Phone: +33 (0)3 20 44 49 98